Digital Display
MKG VAPE TRENDS
A one-time upgrade, Elf Bar hopes that the US FDA will revoke the border detention list, welcome to dialogue!

A wave of one-off regulation in North America has arrived. There is still a 15-day deadline for issuing import alerts and warning letters.
Today, I learned from Haiwai.com that the manufacturer of Elf Bar e-cigarettes hopes that the US FDA will revoke the border detention list! This is also the Chinese manufacturer’s response to the “U.S. FDA’s one-time raid on China.”
It is reported that Imiracle, the manufacturer of disposable e-cigarette brands Elf Bar, Lost Mary and EB Design products, called on the US Food and Drug Administration to revoke the agency’s recent practice of adding Imiracle products to its import red list and impose sanctions on US e-cigarettes. Coherent, clear and apolitical regulation of the market.

IMiracle Shenzhen Technology Co. Ltd. issued a statement regarding the placement of its products on FDA’s Import Alert #98-06. The company said it was disappointed by the FDA’s decision to suddenly and arbitrarily place the company’s product on the FDA Import Red List.
“It was not notified of the decision and was not given an opportunity to address any concerns with the FDA before taking action,” the company said.
It also said the decision also ignores the latest science on e-cigarette use and continues to prevent American adults from using an entire class of nicotine products that the FDA knows are much safer than traditional cigarettes. It also stated that “the inclusion of genuine IMiracle products on the Import Red List and FDA’s simultaneous choice to take no action to reduce the flood of counterfeit products into the U.S. market is a direct result of the agency’s inaction.”
Finally, “IMiracle calls on the FDA to reverse its decision to place IMiracle products on the Import Red List. We welcome engagement and dialogue to create an appropriate and fair regulatory regime around the e-cigarette market that suits all stakeholders.”
This can be said to be the response of Chinese manufacturers after the FDA issued the Import Alert 98-06 list. It is reported that there are many Chinese brands in this list, including brands such as Eonsmoke, Esco Bars and Stik, which are on the agency’s red list.
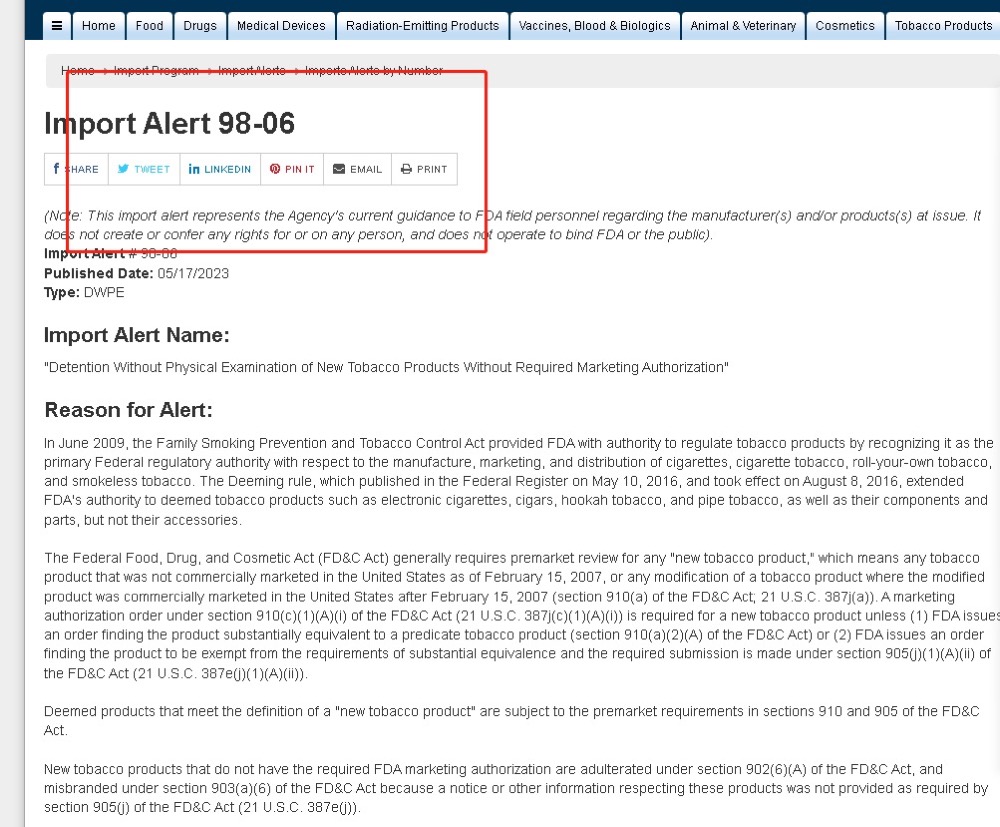
It is understood that once these products have been included in the import red list, they are allowed to be detained at the US port of entry without medical examination.
At present, the US FDA’s raids on many Chinese disposable brands and its “tricks” against disposable e-cigarette companies have also attracted the attention of the industry. Is it a politicized decision by the FDA? Nobody even predicted who would get the next “warning”?
In the U.S. market that Chinese e-cigarette companies have to face, more pressure in the future may not only be limited to competition among peers, but also an important barrier-regulatory risk, which seems to be setting new boundaries for all e-cigarette companies starting line. It is becoming more and more difficult for e-cigarette companies that have not applied for PMTA to develop opportunities in the US market. The establishment of dialogue and communication, and a fair regulatory system should also promote the positive development of the industry.



























