From the “import alert list” to “ordered detention”, the United States has increasingly tightened supervision on whether e-cigarettes can be authorized for marketing.
Concerning today, the U.S. FDA has recently ordered its import inspectors to detain Elf Bar and Esco Bar disposable e-cigarettes from manufacturers and exporters in China and South Korea bound for U.S. ports. These products have been placed on the Import Red List, allowing them to be detained at U.S. ports of entry without a medical examination. The alert was issued by FDA’s Office of Import Operations, part of the Office of Regulatory Affairs (ORA).
Some logistics providers followed suit, mentioning that “EIF Bar and Esco Bar vapes are not accepted in the United States, and the US FDA ordered to detain the goods, which needs attention.”
However, ELF BAR e-cigarettes are now being imported by the FDA in the United States after being caught in the incident of excessive nicotine being taken off the shelves in the United Kingdom.
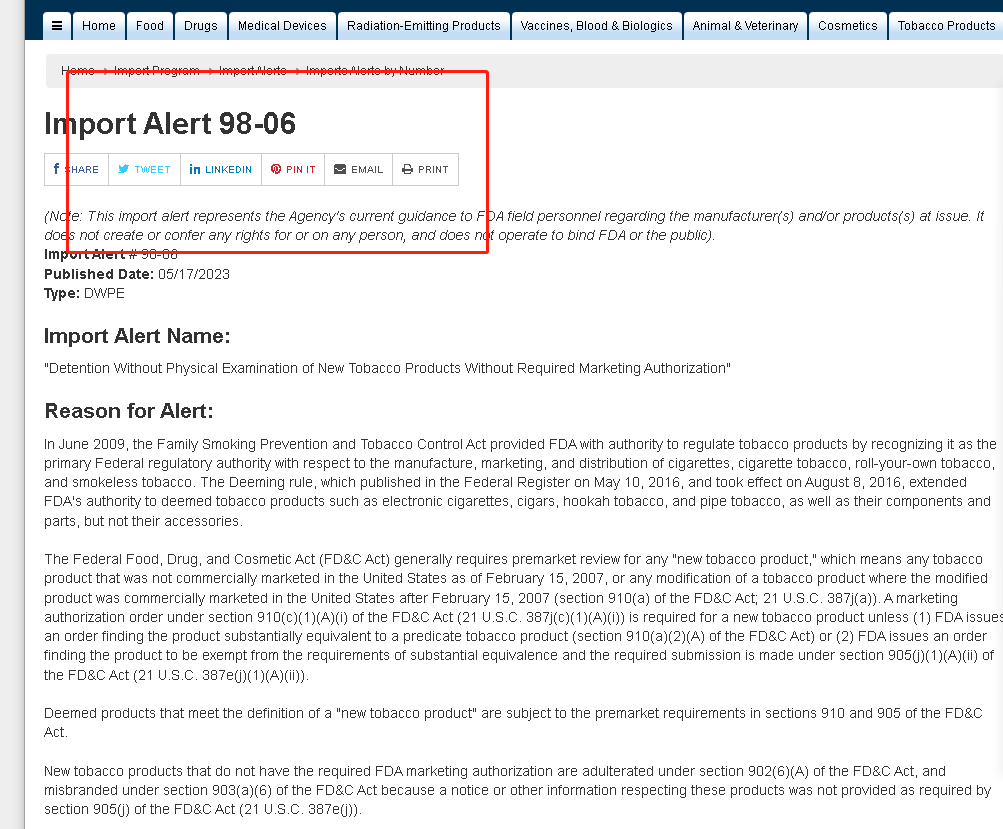
Packages identified as containing Elf Bar or Esco Bar products from red-listed shippers may be detained because they are unauthorized tobacco products, according to the message. The shipper or manufacturer needs to prove that the product is legal before it can be removed from the red list and continue shipping to a U.S. destination. Import Alerts represent current guidance to FDA field personnel. The alert listed Elf Bar under its ELFBAR and EBDESIGN names and named six Chinese shippers who could detain its products, in addition to one from the Republic of Korea and one address in the United States.
It is reported that many Chinese e-cigarette companies have recently been included in the FDA “import alert” list, including ELF BAR. ELF BAR occupies a considerable market share in the North American market, especially in retail such as convenience stores, with extremely high sales.
At the same time, most of the companies in the “Import Warning 98-06” issued by the FDA come from companies in China, the United States, and South Korea, and most of the banned companies are manufacturers, transporters, and distributors of brands.

However, some people questioned whether the FDA’s “enforcement discretion” allowed the agency (out of thousands of brands) to pick two brands with pending PMTAs for enforcement? A major Elf Bar distributor says the FDA could face lawsuits challenging the import ban. And Gregory Conley, Director of Legislative and External Affairs for the American Steam Manufacturers Association, once stated that “unless the Supreme Court or Congress steps in, the FDA will simply double and double its current whack-a-mole enforcement strategy,” “arbitrary selection of products to Additional prohibition is what federal agencies do when they don’t have an effective long-term vision or plan.”
Is there any chance of winning? I remember that in 2009, the FDA Office of Drugs confiscated e-cigarettes shipped from China, triggering a court battle in which the FDA lost the case and the owner of NJOY won.
Speaking of disposable American history, disposable e-cigarettes quickly became popular in the United States after early 2020, when the FDA announced a priority crackdown on pod- and pod-based vaping products sold in flavors other than tobacco and menthol. In the two years since the FDA changed its enforcement focus, disposables accounted for 33% of the convenience store segment of the U.S. vape market.
Regardless, in the U.S., manufacturers may now face delays in product importation and possible legal action by the FDA. The action also highlights growing concerns about potential health risks associated with these products and the importance of ensuring they are marketed and sold in a safe and responsible manner.
In recent years, Chinese enterprises have continuously strengthened independent innovation, actively integrated into the international market, formed unique competitive advantages, brought more new experiences to overseas consumers, and promoted “Made in China” to “Made in China”.
Today, the export of e-cigarettes is expected to be further upgraded in terms of supervision, which needs attention! The U.S. e-cigarette market will also be a game between law enforcement!